Chesapeake Bay Acidification
.........................................................................................................................................
7
There are many biological and biogeochemical influences on carbonate chemistry in Chesapeake Bay,
and these appear to be diverse and widespread. The significant investments made over many decades on
issues such as nutrient discharges into the bay will be very useful for building a robust program on
acidification. However, most of the key processes and pathways that relate directly to coastal ocean
acidification have received little attention to date.
Charge Question C – Given the complexities of coastal ocean acidification, what scientific questions
and information are most urgent for understanding and predicting future changes in Chesapeake Bay?
Unlike in the open ocean where the surface waters closely track atmospheric gas concentrations, coastal
ecosystems and estuaries are strongly influenced by physical, chemical, and biological processes that
affect their chemistry in complicated and significant ways. Shallow waters, variable buffering capacity,
and the influence of biological activities such as photosynthesis and respiration (both aerobic and
anaerobic) drive local pH, pCO
2
, and TCO
2
, and even TA, at regional and local scales. Although many of
these activities are indeed fueled by widespread eutrophication, carbonate dynamics are not directly
explained strictly by increased nutrients in coastal systems. Rather, coastal carbonate dynamics are driven
by a variety of processes and phenomena. For example, in estuaries fluxes of carbon across system
boundaries such as air:water, land:estuary, ocean:estuary, and sediment:water can affect pCO
2
and pH in
estuarine waters (Fig. 1).
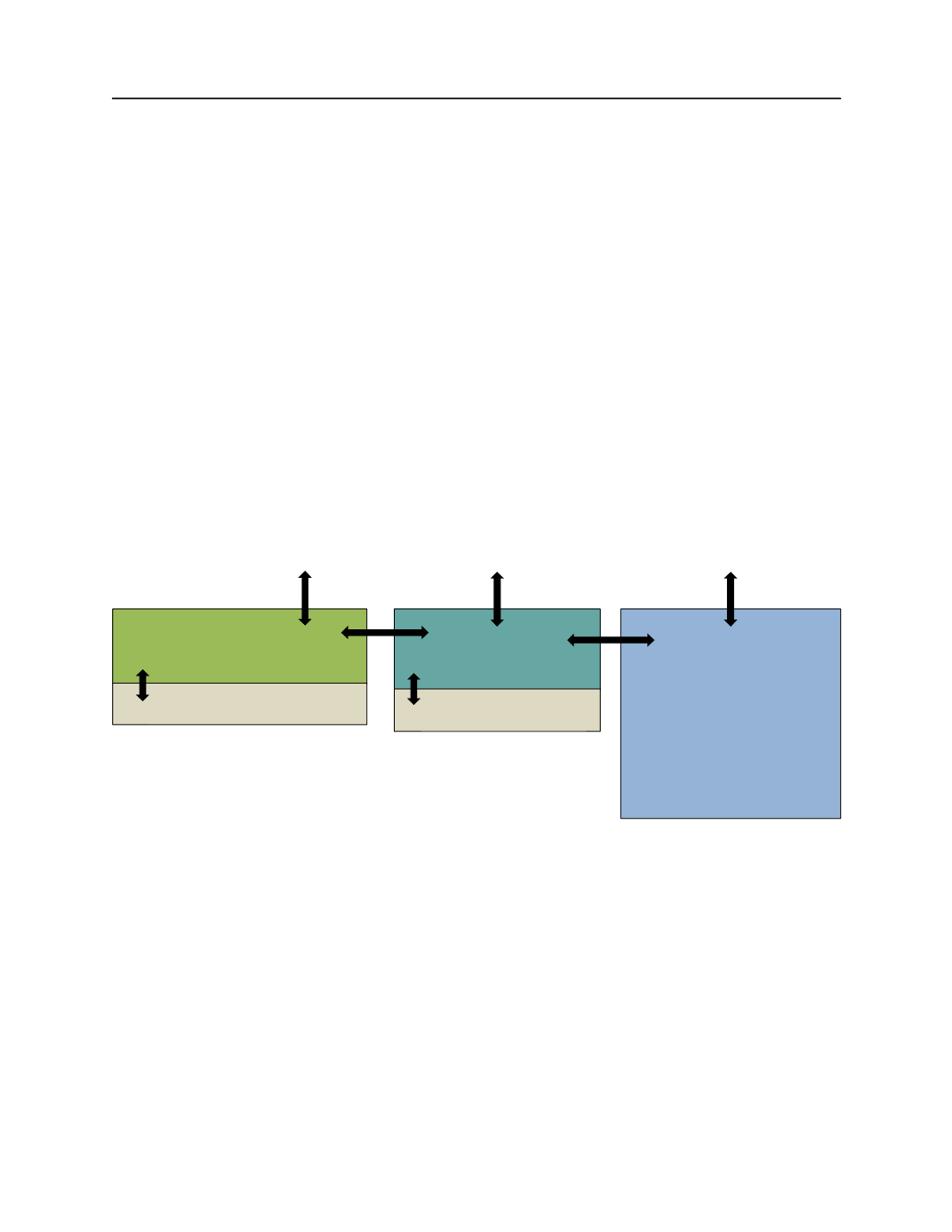
Figure 1. Simplified Conceptual Model.
Key ecosystem components and the interfaces between them that
regulate carbonate chemistry in estuarine and coastal landscapes. Arrows represent potential linkages across which
CO
2
and/or TCO
2
can move (e.g., air:water, land:estuary, ocean:estuary, sediment:water). Arrow size does not
connote extent of flux. Observing systems and efforts should focus explicitly on measuring flux rates across these
subsystems and be designed to detect how these fluxes are changing through time.
A greater understanding of the relative importance of carbon fluxes (pCO
2
, TCO
2
) across ecosystem
boundaries will be vital for modeling coastal carbonate chemistry and for forecasting how rising CO
2
may
influence acidification of the Bay directly and, perhaps more importantly, indirectly through biological
and biogeochemical processes. The flux of CO
2
across the air:sea interface is well understood in open
ocean surface waters, and when combined with rates of atmospheric CO
2
concentration increase, is the
basis on which acidification predictions are formulated. Although the atmosphere is pushing increasingly
harder on all surfaces and ecosystems of the globe, it is not yet clear how increased atmospheric pCO
2
will propagate through the ecological subsystems of the Chesapeake Bay and other coastal ecosystems.
Measurements that enable such fluxes to be quantified will be vital for generating predictive models about
Terrestrial
& Riverine
Estuary
Ocean
Atmosphere
Soils/Sediments
Sediments


